Project information
OASIS aims to develop an antimicrobial resistance (AMR) surveillance strategy in a One Health context, and applicable in high-, middle-, and low-income countries. The proposed strategy challenges the strong reliance on laboratory-based AMR surveillance for meeting objectives of the Global Action Plan on AMR .
Inappropriate use of antibiotics is a key driver for AMR. Antimicrobial stewardship programmes aim at reducing inappropriate use by promoting evidence-based prescribing of antimicrobial drugs. Knowledge of the prevalence of AMR is central to this evidence base, and therefore for the design and implementation of antimicrobial stewardship programmes. The AMR prevalence estimates in the human and veterinary domains suffer from the same drawbacks: selection bias in isolates submitted for antibiotic susceptibility testing, and absence of locally-relevant information due to aggregated data on (sub-)national level. The required evidence base needs population-based AMR prevalence surveys. However, conventional surveys are time consuming, expensive, and preclude the identification of local variations in AMR prevalence, given the limitations in sample size.
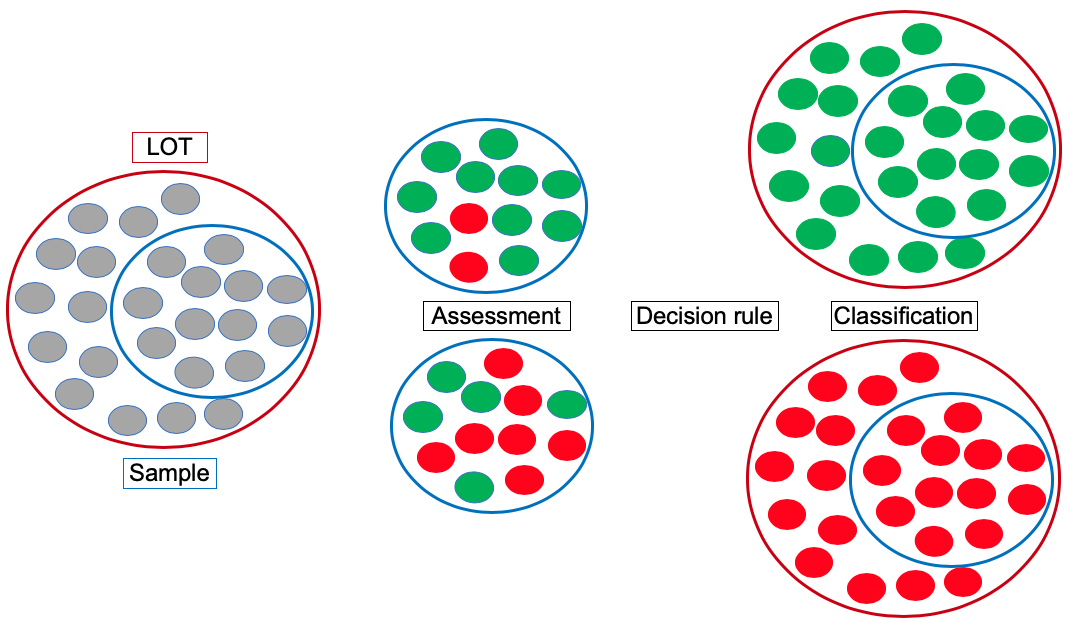
OASIS moves from conventionally estimating AMR prevalence to classifying populations/settings as having a “high” or “low” AMR prevalence, by applying a Lot Quality Assurance Sampling (LQAS) approach. This requires much smaller sample sizes, and is uniquely positioned for population-based AMR surveillance.
The project validates the LQAS-based AMR surveillance approach against conventional AMR prevalence surveys in the veterinary domain for the first time. It additionally provides the evidence that aggregation of LQAS results (which are classifications of “high” or “low” AMR prevalence), can serve as a valid estimate of conventional AMR prevalence survey results (which are prevalence estimations). It is especially this latter validation step that will make LQAS-based AMR surveillance an attractive strategy for the health profession and policy makers alike, as the LQAS-based results provide the required local evidence for appropriate empirical antimicrobial treatment, while the aggregation of these results in a conventional AMR prevalence estimate provides policy makers with a tool for measuring the impact of interventions that are aimed at reducing AMR prevalence at district, regional, or national levels.
OASIS’ implementation research component engages domain-specific stakeholders throughout the project to optimise knowledge utilisation, and facilitate the translation of results into policy.